Ominedo History Chronological table
Ominedo is a long-established manufacturer founded in 1900. We can maximize the power of natural products by using supercritical (Co2), alcohol, and hot water extraction techniques as well as manufacturing techniques which have been sophisticated since the founding of the company.
- Stage 1: Founding stage: 1887-1945
- Stage2: Postwar development: 1945-1975
- Stage 3: Business development in new markets: 1976-1990
- Stage 4: Further advancements: 1990-2014
Stage 3: Business development in new markets: 1976-1990
-
1976

Shigenori Tsuji appointed as Chief Executive Officer
-
1976

Research members dispatched to the Institute of Natural Medicine, Toyama Medical and Pharmaceutical University
-
1976

Ethical Kampo products listed in NHI Medicine Price Standard
-
1977

“Omine Ichogan” went on sale
-
1979

Shigeko Tsuji appointed as Chief Executive Officer
-
1981

Company started OEM of ethical Kampo products
-
1983

Annual sales exceeded 1 billion yen
-
1984

“Hojugan” went on sale
-
1986

Twenty-seven ethical Kampo products started to be supplied to medical institutions in Japan through Kanebo
-
1986

“Keifugan” went on sale
-
1987

Capital increased to 20 million yen
Nine ethical Kampo products added -
1987

OTC Kampo products launched in the market after changing specifications (40 products)
-
1988

Additional quality management building and extraction plant built
-
1988

“Taihoshin Gold” went on sale
-
1989

Kashihara Research Center and crude medicine warehouse newly built
-
1989

Annual sales exceeded 2 billion yen
1976

Shigenori Tsuji appointed as Chief Executive Officer
While Shigenori received education on how to become a leader from Seiroku, who was
his father and the President of the company, and gained deep knowledge about medicines,
especially Kampo products, he mainly demonstrated an ability to develop new sales
routes. It was Shigenori who paved the way for Ominedo to sell products to pharmacies
which in turn expanded business performance in the development of products for the
home.
1976

Research members dispatched to the Institute of Natural Medicine, Toyama Medical
and Pharmaceutical University
Our research members were dispatched to the research office of Professor Zenichi Ogita
from Toyama Medical and Pharmaceutical University on a referral from Professor
Shieru Arichi from Kinki University. They conducted research on oriental medicine on a
full scale including research on musk and peony root and pharmacological and genetic
research of Hachimijiogan using raw materials which were previously used at Ominedo.
1976
Ethical Kampo products listed in NHI Medicine Price Standard
Thanks to the efforts of people including mainly Dr. Taro Takemi, the president of the
Japan Medical Association, many ethical Kampo products were listed in the NHI Medicine
Price Standard. This led to an increased interest in Kampo products in medical
institutions. In response to this, our company also established the “ethical product
development project.” We steadily proceeded with preparations including selection of
prescriptions, determination of dosage form, establishment of applications, sales
strategies, etc.
1977

“Omine Ichogan” went on sale
In the beginning of the 1970s, “Gyojagan,” a phellodendron bark product that further
improved “En no Gyoja Sodenyaku Gyoja Daranisuke,” was a mainstream product, but
it developed further into “Omine Ichogan.” Common Daranisuke is a medicine for improving
the condition of the abdomen and intestines, but “Omine Ichogan” contains peony root
as an analgesic-antispasmodic medicine and aims to relieve “gastric pain,” which are its
main characteristics. It had a good reputation at research centers and hospitals, and as
a result of additional applications of the effects based on clinical literature in 1977 and
clinical assessment results from three institutions in 1993, “gastric pain” and “gastric
discomfort” were added as effects. “Omine Ichogan” contains powdered phellodendron
bark, powdered glycyrrhiza, powdered peony root, and powdered ginseng.
1979

Shigeko Tsuji appointed as Chief Executive Officer
In deep grief of the accidental death of the President, Shigenori, Shigeko Tsuji decided
to take over his position. One of the policies she enacted was “thorough education of
employees,” and she aggressively performed internal organizational change. In 1995,
she received an award from the governor as a contributor in pharmaceutical affairs. It
was the award to show her great personality such as the way she was very calm and
polite and had great prestige among her employees and business partners.
1981

Company started OEM of ethical Kampo products
The ethical product development project which began in 1976 produced results, and in
1981, 24 ethical Kampo products were listed in the NHI Medicine Price Standard. The sales
of thse products were entrusted to Kanebo Pharmaceutical Co., Ltd., and Kanebo
started to supply the products to medical institutions throughout Japan.
1984

“Hojugan” went on sale
Based on “Hachimijiogan,” “Hojugan” pills were made by kneading powdered crude
medicines with honey. It is used mainly in middle-aged people in following circumstances:
・A person who gets thirsty and feels like urinating, often waking up at night to urinate.
Also, a person who cannot urinate smoothly and still feels like urinating even after
urination.
・An older person who has dry skin without moisture and sometimes feel itchiness.
・A person who is physically heavily exhausted, feels dull with cold and tired feet, and
has a hot feeling in the sole of his or her feet, but has no diarrhea or vomiting.
1986
Twenty-seven ethical Kampo products started to be supplied to medical institutions in
Japan through Kanebo
At the start of the supply of the products, a phrase, “Ominedo’s,” was added to the head
of the product trademark. Thereafter, we had a chance to change the product
specifications, etc., and concerning the trademark, Kanebo requested Ominedo to bring
Kanebo’s name to the forefront as its sales strategy. In 1986, this change was reflected
in all products listed in the NHI Medicine Price Standard.
1986

“Keifugan” went on sale
Based on “Keishibukuryogan,” “Keifugan” pills were made by kneading powdered crude
medicines with honey. It contains cinnamon bark, poria sclerotium, moutan bark, peach
kernel, and peony root.
Effective against: The following symptoms with occasional complaints of lower
abdominal pain, shoulder pain, heavy-headedness, dizziness, and cold feet with hot
flashes in a person who has relative physical strength:
Irregular menstruation, menstrual disorder, menstrual pain, menopausal disorder,
climacteric disorder, shoulder pain, dizziness, heavy-headedness, bruising, chilblains,
chloasma
1987
Capital increased to 20 million yen
Nine ethical Kampo products added
Nine ethical Kampo products were added, and a total of 36 products began to be
provided to medical institutions in Japan via Kanebo. These 36 products are still being
supplied via Kracie Pharmaceutical, Ltd.
1987



OTC Kampo products launched in the market after changing specifications (40
products)
The OTC Kampo product series was launched in the market after changing their
specifications. The products were launched with the design that Seiroku Tsuji initially
used. This design was awarded by the governor at the “3rd Nara Good Design Exhibition”
(1991).
1988
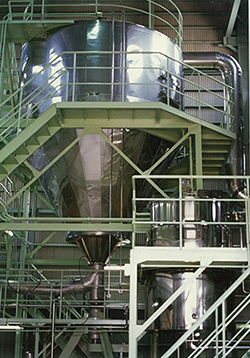
Additional quality management building and extraction plant built
The quality management laboratory and extraction factory were expanded. The
extraction factory was expanded to become four times larger than it used to be, and a
spray dryer which was the latest model at that time (a machine to manufacture
powdered extracts by brewing Kampo medicine and drying and powdering the extracted
solution) was introduced.
1988

“Taihoshin Gold” went on sale
In connection with the Washington Convention, unification of the ingredient amount of
musk-containing products and of the effects were conducted in the entire industry.
Ominedo changed the ingredient amount of musk contained in “Taihoshin,” and its
effectiveness was changed to “palpitations, shortness of breath, and restoring
consciousness” as unified in the industry.
1989


Kashihara Research Center and crude medicine warehouse newly built
The Kashihara Research Center and crude medicine warehouse were newly built. In
addition, a crude medicine sample room was opened in the research center.
In this crude medicine exhibition area, about 1,500 kinds of crude medicine samples were
displayed at all times. Our company’s products, real constituent crude medicines, and
product samples were also exhibited.










